
ZYRTEC® Clinical Data
See the evidence—and make ZYRTEC® your foundation for allergy relief
Data show ZYRTEC® provides effective symptom relief in both indoor and outdoor allergies, is generally well tolerated, and significantly improved allergy-related quality of life.1*†‡
- Starts working 2 hours faster than Claritin® the first day patients take it (ZYRTEC® at hour 1 and Claritin® at hour 3)2,3§
- Delivered 33% greater relief than Allegra® at 21 to 24 hours4||
- Provides consistent symptom relief in both seasonal allergic rhinitis and perennial allergic rhinitis5
- Relieves ocular symptoms6
- Significantly improved allergy-related quality of life1*†‡


See pediatric clinical results
Efficacy and tolerability findings in different age groups.

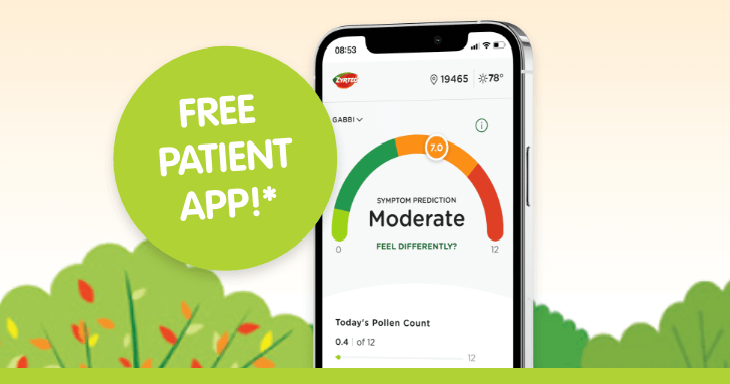
Free app helps patients manage allergies*
Symptom tracking, pollen forecasts, and more with ZYRTEC® ALLERGYCAST®.
*Standard data rates apply.
*Randomized, placebo-controlled, 2-week study of adult patients with seasonal allergic rhinitis (N=431 for each treatment group).
†Impact on self-reported work/school-related productivity and activity impairment was assessed using the Work Productivity and Activity Impairment-Allergy Specific (WPAI-AS) questionnaire, which measures performance based on impairment at work (limitations in the amount or kind of work done, work accomplished, or work done as carefully as usual) and activity impairment (limitations in usual activities, such as work around the house, shopping, child care, exercising, etc).
‡Patient disease-specific quality of life was assessed using the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ), which measures 7 domains: activity limitation, sleep problems, nose symptoms, eye symptoms, non–hay fever symptoms, practical problems, and emotional function.
§ZYRTEC® 10 mg starts working at hour 1 and Claritin® starts working at hour 3, based on first dose on the first day of a 2-day study in 2 pollen-chamber studies. Primary endpoint measured mean improvement from baseline in Major Symptom Complex (MSC) severity score. MSC symptoms included runny nose, sniffles, itchy nose, nose blows, sneezes, and watery eyes.
||Based on first dose on the first day of a 2-day pollen-chamber study with ZYRTEC® 10 mg vs Allegra® 180 mg at hours 21 to 24. Primary efficacy endpoint was change in total symptom severity score from baseline at hours 21 to 24. Total symptom severity complex score was defined as the sum of self-assessed severity scores of 4 symptoms: runny nose, sneezing, itchy nose/palate/throat, and itchy/watery eyes.
References: 1. Murray JJ, Nathan RA, Bronsky EA, Olufade AO, Chapman D, Kramer B. Comprehensive evaluation of cetirizine in the management of seasonal allergic rhinitis: impact on symptoms, quality of life, productivity, and activity impairment. Allergy Asthma Proc. 2002;23(6):391-398. 2. Day JH, Briscoe M, Widlitz MD. Cetirizine, loratadine, or placebo in subjects with seasonal allergic rhinitis: effects after controlled ragweed pollen challenge in an environmental exposure unit. J Allergy Clin Immunol. 1998;101(5):638-645. 3. Day JH, Briscoe M, Rafeiro E, Chapman D, Kramer B. Comparative onset of action and symptom relief with cetirizine, loratadine, or placebo in an environmental exposure unit in subjects with seasonal allergic rhinitis: confirmation of a test system. Ann Allergy Asthma Immunol. 2001;87(6):474-481. 4. Day JH, Briscoe MP, Rafiero E, Hewlett D, Chapman D, Kramer B. Randomized double-blind comparison of cetirizine and fexofenadine after pollen challenge in the environmental exposure unit: duration of effect in subjects with seasonal allergic rhinitis. Allergy Asthma Proc. 2004;25(1):59-68. 5. Data on file. Johnson & Johnson Consumer Inc., McNeil Consumer Healthcare Division. 6. Patel M, Urdaneta E, Franklin K, et al. Cetirizine significantly relieves ocular allergy symptoms in subjects with seasonal allergic rhinitis. Poster Abstract. Data on file. Johnson & Johnson Consumer Inc., McNeil Consumer Health Division.
