Pediatric Clinical Data
Give your pediatric patients significant allergy relief with Children’s ZYRTEC®1
Studies show powerful symptom responses in ages 2 to 111
ZYRTEC® is generally well tolerated by children1*
In clinical trials in children with seasonal allergic rhinitis (SAR), ZYRTEC® demonstrated:
- Effective symptom relief in studies of children ages 2 to 6 and ages 6 to 11
- Consistent improvement in both age groups
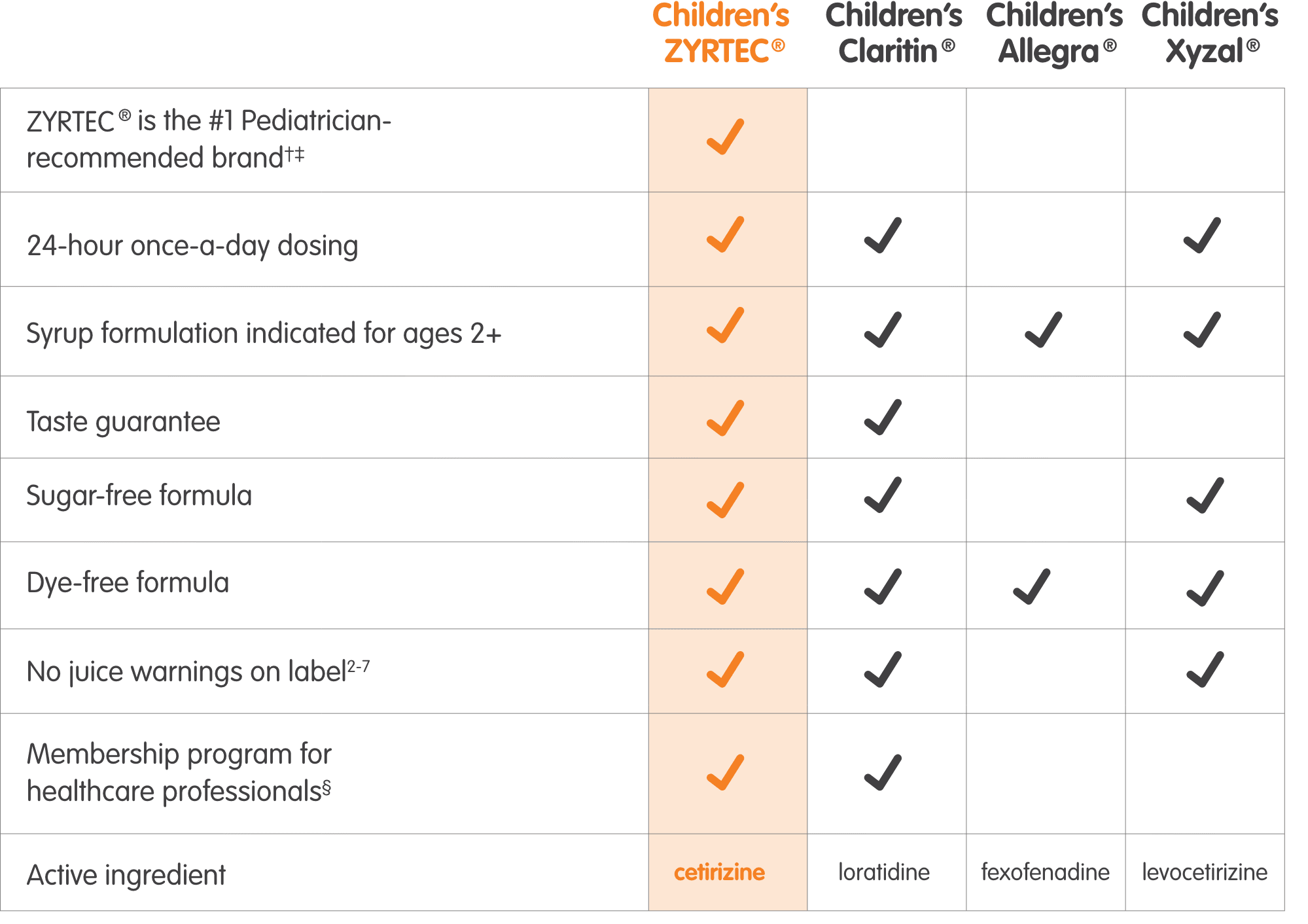
See how Children’s ZYRTEC® compares with other children’s OTC oral antihistamines

Good taste, guaranteed
Children’s ZYRTEC® is covered by the Kenvue Pediatrics Taste Guarantee.


A story for caregivers to share with kids
Popular 2-part set helps families manage allergies. Available to download in English and Spanish.


Free monthly Bundle Box supports your pediatric patient care
Samples and resources from Children’s ZYRTEC® and other Kenvue Pediatric products.
*Tolerability profile demonstrated in clinical trials of children ages 6 to 11 years.
†Among OTC oral antihistamines.
‡ZYRTEC® is the #1 Pediatrician-recommended brand.
§ZYRTEC® membership program for healthcare professionals is ZYRTEC ZIPLINE®.
References: 1. Data on file. Johnson & Johnson Consumer Inc., McNeil Consumer Health Division. 2. Children’s Allegra® Drug Facts. 3. Children’s Claritin® Oral Solution Drug Facts. 4. Children’s ZYRTEC® Allergy Syrup Drug Facts. 5. Xyzal® Allergy 24HR Oral Solution Drug Facts. 6. Allegra® [package insert]. Bridgewater, NJ: Sanofi-Aventis U.S. LLC; 2007. 7. FAQs. Allegra®. Accessed May 20, 2021. https://www.allegra.com/en-us/faq-what-are-seasonal-allergies/
